Aza-ortho-quinone methides (aza-o-QMs) are highly reactive intermediates that have found valuable applications in many areas of chemistry and biology. Traditional synthetic methods for the in situ formation of highly reactive aza-o-QMs mainly rely on pyrolysis, UV photolysis, base and Brønsted acid promoted β-elimination or tautomerization.
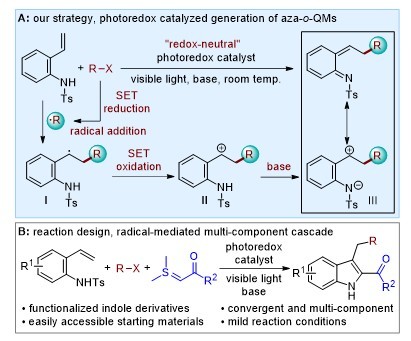
Recently, visible light photoredox catalysis has been identified as a powerful tool for the generation of various radicals and radical ions under remarkably mild conditions. Notably, these radical intermediates can allow facile incorporation of diverse synthetically and medicinally important functionalities into alkenes in a controlled way. Recognizing the importance of this reactivity mode as well as the neutral and zwitterionic characteristics of aza-o-QMs, we explored the visible light photoredox-catalyzed and radical-mediated strategy for in situ generation of aza-o-QMs from easily accessible alkene feedstocks, such as alkenylanilines under ambient conditions. This strategy allows us to further expand the chemistry of aza-o-QM chemistry. For example, this protocol enables an efficient multicomponent cascade reaction of alkenylanilines, halides and sulfur ylides, providing convergent and streamlined synthesis of diversely functionalized indole heterocycles in a single flask operation. Many of our ongoing projects are based on this method.
Selected references:
1. Y.-Y. Liu, X.-Y. Yu, J.-R. Chen*, M.-M. Qiao, X. Qi, D.-Q. Shi, W.-J. Xiao*, Angew. Chem. Int. Ed. 2017, in press, doi: 10.1002/anie.201704690.
2. X.-Y. Yu, J.-R. Chen*, Q. Wei, H.-G. Cheng, Z.-C. Liu, W.-J. Xiao, Chem. Eur. J. 2016, 22, 6774-6778.
|